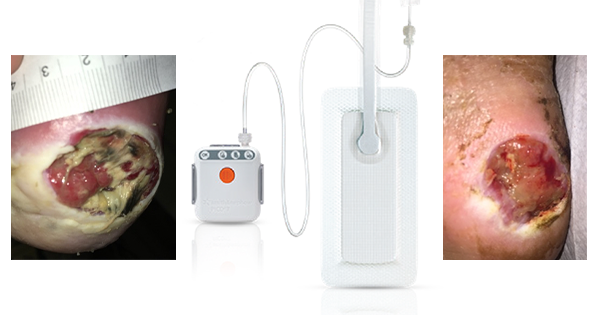
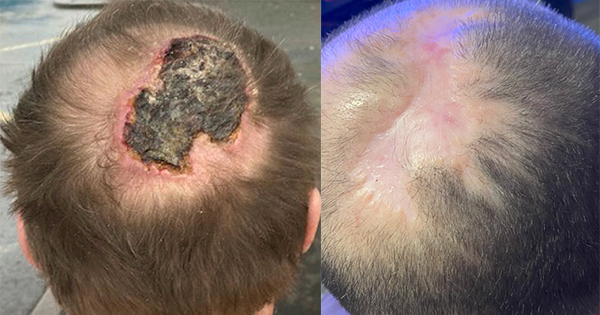
Collagen is a key component of the extracellular matrix (ECM) and plays a critical role in all phases of wound healing. There are a number of advanced wound care dressings available that incorporate collagen to enhance wound bed preparation. Some comprise type I collagen and may be combined with other ingredients such as alginates or oxidised regenerated cellulose (ORC). This article focuses on the use of fish-derived type I collagen dressings in the treatment of stalled traumatic and surgical wounds or hard-to-heal chronic ulcers.
Advances in wound dressings
Over the last 30 years there has been a shift from traditional wound dressings towards those advanced therapies that aim to optimise the wound healing environment (Enoch and Harding, 2003). In more recent years, wound care products have been developed that aim to replicate or add to the ECM. The ECM is the major component of the dermis and provides a structural support for cells, growth factors and receptors that are essential to wound healing (International Consensus, 2011).
With a wide range of dressings to choose from, dressing selection is a significant challenge for wound care clinicians. Increased costs and the need for evidence-based treatment approaches are creating a demand for well-designed randomised controlled trials to support purchase and use (Rudnick et al, 2006). The ideal dressing should achieve rapid healing at reasonable cost with minimal inconvenience to the patient (Boateng et al, 2007).
What is collagen?
Collagen is the most abundant protein in the human body and is a major component of the ECM. It comprises three polypeptide chains that are rich in hydroxyproline amino acids (Mian et al, 1992) and are twisted together into a triple-helical structure.
Over 20 different types of collagen have been identified in humans; the main types are type I, II and III and together they make up 80% of the body’s collagen. Type I and III are important for wound healing (Li et al, 2007).
Role of collagen in wound healing.
In a healing wound a cascade of events occurs that includes platelet accumulation, inflammation, fibroblast proliferation, cell contraction, angiogenesis and re-epithelialisation (Moore, 2010), ultimately leading to scar formation (Rudnick, 2006) and wound remodelling (Brett, 2008).
Collagen plays an important role in each of these phases of wound healing due to its chemotactic role. It attracts cells such as fibroblasts and keratinocytes to the wound. This encourages debridement, angiogenesis and re-epithelialisation (Weber et al, 1984; Zbigniew, 2003).
A chronic wound is stalled at one of these healing stages (Vowden, 2011). This usually occurs during the inflammatory phase and is linked to elevated levels of matrix metalloproteinases (MMPs) in the wound (Singh et al, 2011; Gibson et al, 2009). In normal wound healing, proteases such as MMPs are attracted to the wound during the inflammatory phase and have an important role in breaking down unhealthy ECM so that new tissue forms (Parks, 1995; Jeffrey, 1995). However, when MMPs are present in a wound at elevated levels for a prolonged period of time, this results in the destruction of healthy ECM, which is associated with delayed wound healing and an increase in wound size (Vowden, 2011).
When the excess of MMPs is not balanced by normal physiological processes, alternative methods are required to reduce protease levels in the wound. This suggests a role for dressings containing collagen in the management of wounds where healing is stalled (Rangaraj et al, 2011).
What are collagen dressings?
Native intact collagen provides a natural scaffold or substrate for new tissue growth (Ruszczak, 2003). Dressings containing collagen are thought to provide the wound with an alternative collagen source that can be degraded by the high levels of MMPs as a sacrificial substrate, leaving the endogenous native collagen to continue normal wound healing (Brett, 2008).
There are a number of different collagen dressings available that use a variety of carriers and combining agents such as gels, pastes, polymers and oxidised regenerated cellulose (Brett, 2008). The collagen contained in these products also varies in type and source. Certain dressings contain native (type I) collagen in which the triple helix formation is intact; others contain denatured or reconstituted collagen, which is referred to as gelatin.
Most collagen dressings contain collagen derived from bovine and porcine sources (Vivas et al, 2011). Although these collagens are purified, there remains a theoretical concern regarding the potential for prion diseases such as bovine spongiform encephalopathy (BSE) (Liu et al, 2006; Jabar, 2011). There have also been concerns regarding the integration of porcine collagens into scar tissue (Burns et al, 2010), while cultural/religious issues may prevent their use on some patients (Jabar, 2011). Human-derived collagens are linked with fewer immunological concerns, however they tend to be more expensive than animal-derived collagens (Vivas et al, 2011). More recently collagen derived from fish has been proposed as a cost-effective source of collagen for use in dressings.
What is piscean collagen?
Piscean collagen is a protein extracted from fish waste, including the scales, skin or air bladders, which is predominantly sourced from fresh water carp, Labeo rohita (Pati et al, 2010). Purifying collagen from fish involves: alkaline hydrolysis, returning the collagen to a neutral pH and subjecting it to acid hydrolysis to stabilise it. The collagen is freeze-dried into particles, sponge and sheets that can be cut into the required shape and size (Basha et al, 2011). The film membrane preparation is cast onto slabs and is not freeze-dried.
The extraction process for piscean collagen preserves its triple-helix structure. Dressings containing piscean collagen have demonstrated a high level of biocompatibility, while animal studies have found that collagen extracted from fish did not produce an immunological response or allergic reactions and was comparable to type I bovine collagens (Pati et al, 2010).
AVAILABILITY OF type I PISCEAN collagen products
In the UK a number of fish-derived collagen products are available (Table 1). These products are non-toxic, non-allergenic, non-immunogenic and non-pyrogenic.
To choose the most appropriate piscean collagen dressing, selection should be approached with care. All dressings are biodegradable, although there may be some variation in the time they take to degrade in the wound. Variation also applies to the absorption capacity of the dressings and in their ability to mould to the contours of the wound. A particle method of delivery (Helisorb® Particles, Medira Ltd) increases the activity of the collagen by providing an increased surface area and allowing intimate contact with the contours of the wound bed. The particles may be used in infected wounds in combination with appropriate antimicrobial or systemic treatment. All other available piscean products can be used once infection has resolved.
Tip: The particles may be mixed with saline to form a paste for ease of use and to maintain a moist wound environment

WHEN AND HOW to USE type I PISCEAN collagen dressings
Piscean collagen dressings are currently used as second-line dressings in non-healing wounds that have failed to respond to other treatment approaches or split skin grafting. They are haemostatic and may provide relief from pain. Other indications are described in Table 1. Collagen products should not be used on patients sensitive to collagen from any source.
How to apply the dressings
First ensure the wound bed is thoroughly prepared according to local protocols. Apply to the wound bed and keep the area moist. A secondary non-adherent dressing (e.g. paraffin gauze, TelfaTM [Kendall] or MepitelTM [MÖlynlycke]) should be applied over the collagen dressing. An absorbent layer (e.g. gauze) is then placed on top. Finally the dressing should be secured using an appropriate film or crepe dressing.
Tip: When applying Neuskin-FTM, it can curl up at the edges. Simply wet the sheet edges with saline on the fingertips to improve the contact area
How frequently should dressings be changed?
Exudate levels may increase due to healing and the inflammatory response. The authors recommend initial dressing changes two to three times per week, depending on the exudate level. Remaining particles in the wound should be rinsed off. Overgranulation may occur with collagen dressings. If this is present, the collagen product can be discontinued and the overgranulation tissue treated for two weeks using a 1% hydrocortisone cream.

EVIDENCE FOR type I PISCEAN collagen dressings
The use of collagen dressings is supported by relatively sparse data, with much of the evidence derived from non-comparative case reports and clinical experience.
Piscean collagen has been used successfully to treat canine wounds, resulting in normal cell proliferation after treatment (Takai et al, 1997). In a study using a comparable bovine type I collagen matrix dressing faster healing rates were reported in 15 patients with postoperative wounds when compared to conventional treatment (6.1 vs 9.4 weeks for controls) (Kolenik et al 1999).
Anecdotal evidence to date using type 1 piscean collagen products has demonstrated that they are a useful adjunct to facilitate healing in patients with chronic ulcers (including diabetic foot and pressure ulcers), dehisced surgical wounds and donor site wounds (Medira Ltd, data on file). In addition, faster healing rates have been observed in patients with epidermolysis bullosa when using Helisorb® Particles as a primary contact layer. The product was easy to apply and impacted minimally on the patients’ normal dressing regimen (DeLuca, clinical data).
Cost benefits of using collagen dressings
The potential advantages of advanced wound care products have been summarised by Vowden (2011). These include the potential to improve healing rates, reduce symptoms and improve quality of life together with a reduction in healthcare costs. Advanced therapies may add to the initial cost burden of care. It is therefore important that they are used judiciously following accurate assessment in order to avoid inappropriate application or use in inappropriate circumstances.
Summary
This made easy supplement provides a focus on collagen dressings derived from type I piscean collagen. Fish-derived collagen products are able to deliver a wide range of benefits to the wound healing process. When applied topically, the collagen acts as a haemostat and helps to stimulate the growth of new tissue in the wound bed. Piscean collagen appears to have a number of advantages over mammalian derived collagen, including decreased cost and fewer adverse immunological reactions. This advanced wound care intervention has the potential to improve healing rates, reduce symptoms and improve quality of life while reducing long-term healthcare costs.
AUTHOR DETAILS
Westgate S[1], Cutting KF[2], DeLuca G[3], Asaad K[4]
1. Project Manager and Research Scientist, Perfectus Medical, UK
2. Director, Perfectus Medical, UK
3. Clinical Nurse Specialist, Plastic Surgery, St Thomas’ Hospital, London
4. Clinical Fellow, Plastic Surgery, St Thomas’ Hospital, London
References
- Basha SK, Kumar RVS, Haragopal V et al (2011). Effects of fish scales extracted collagen biocastings oncutaneous wound healing in dogs. Res J Pharma Biol Chem Sci 2: 36-49.
- Boateng JS, Matthews KR, Stephens HNE (2007). Wound healing dressings and drug delivery systems: A review. J Pharm Sci 97(8): 2892-923.
- Bradley M, Cullum N, Nelson EA, et al (1999). Systematic reviews of wound care management: (2). Dressings and topical agents used in the healing of chronic wounds. Health Technol Assess 3: 1-35.
- Brett D (2008). A review of collagen and collagen based wound dressings. Wounds 20(12): 347-53.
- Burns NK, Jaffari MV, Rios CN, et al (2010). Non cross-linked porcine acellular dermal matrices for abdominal wall reconstruction. Plast Reconstr Surg 125: 167-76.
- Enoch S, Harding K (2003). Wound bed preparation: the science behind the removal of barriers to healing. Wounds 15: 213-29.
- Gibson D, B Cullen, R Legerstee, Harding KG and Schultz G. MMPs Made Easy. Wounds International 2009; 1(1). Available from: http://www.woundsinternational.com
- International Consensus. Acellular matrices for the treatment of wounds. Wounds International 2011. Available from http://www.woundsinternational.com
- Jabar NA (2011). Extraction of collagen from fish waste and determination of its physico-chemical characteristics Bachelor of Science (Hons.) Final Year Project Universiti Teknologi MARA.
- Jeffrey J (1995). Metalloproteinases and tissue turnover. Wounds 13-22.
- Kolenik SA, McGovern TW, Leffell DJ (1999). Use of lyophilized bovine collagen matrix in postoperative wound healing. Dermatol Sci 25: 303-07.
- Li J, Chen J, Kirsner R (2007). Pathophysiology of acute wound healing. Clin Dermatol 25: 9-18.
- Lui Y, Griffith M, Watsky MA, et al (2006). Properties of porcine and recombinant human collagen matrices for optically clear tissue engineering applications. Biomacromolecules 7: 1819-28.
- Mian M, Beghe F, Mian E (1992). Collagen as a pharmacological approach in wound healing. Int J Tissue React 14(Suppl): 1-9.
- Montesano R, Orci L, Vasselli P (1983). In vitro rapid organisation of endothelial cells into capilary-like networks is promoted by collagen matrices. J Cell Biol 97: 1648-52.
- Moore K (2010). Cell biology of normal and impaired healing. In: Percival S, Cutting KF (eds.) Microbiology of Wounds. Boca Raton, FL: CRC Press.
- Parks WC (1995). The production, role and regulation of matrix metalloproteinases in the healing epidermis. Wounds 7: 23-37.
- Pati F, Dhara S, Adhikari B (2010). Fish collagen: A potential material for biomedical application. Student’s Technology Symposium (TechSym), 34-8.
- Rangaraj A, Harding K, Leaper (2011). Role of collagen in wound management. Wounds UK 7(11): 54-63.
- Rudnick A (2006). Advances in tissue engineering and use of type 1 bovine collagen particles in wound bed preparation. J Wound Care 15: 402-04.
- Ruszczak Z (2003). Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev 55(12):1595-611.
- Singh O, Gupta SS, Soni M, et al (2011). Collagen dressing versus conventional dressings inburn and chronic wounds: A retrospective study.
- J Cutan Aesthet Surg 4: 12-6.
- Takai M, Shimizu Y, Shimizu J, et al (1997). Wound healing composition using squid chitin and fish skin collagen. Japan patent application 669,956.
- Vivas A, Tang JC, Kirsner RS (2011). Collagen-based tissue-engineered composites. J Wound Technol 13: 62-67.
- Vowden, P (2011). Hard-to-heal wounds Made Easy. Wounds International. Available from: http://www.woundsinternational.com
- Weber L, Kirsch E, Muller P, et al (1984). Collagen type distribution and macromolecular organization of connective tissue in different layers of human skin. J Inv Derm 82:156-60.
- Zbigniew R (2003). Effect of collagen matrices on dermal wound healing. Adv Drug Del Rev 55: 1595-611.
Supported by Medira Ltd
For further information please go to:
www.medira.co.uk